Fab-TACS® Trial Kit Request

The Fab-based traceless affinity cell selection (Fab-TACS®) technology represents an affinity chromatography system for non-magnetic isolation of exosomes. It is based on Twin-Strep-tagged CD-speci c Fab fragments (Fabs), which reversibly capture and release the exosomes. The technology delivers label-free exosomes with intact biological functions in a standardized manner with highly reproducible quality. The exosomes can be isolated from different cell culture supernatants, serum and plasma.
Simple Workflow
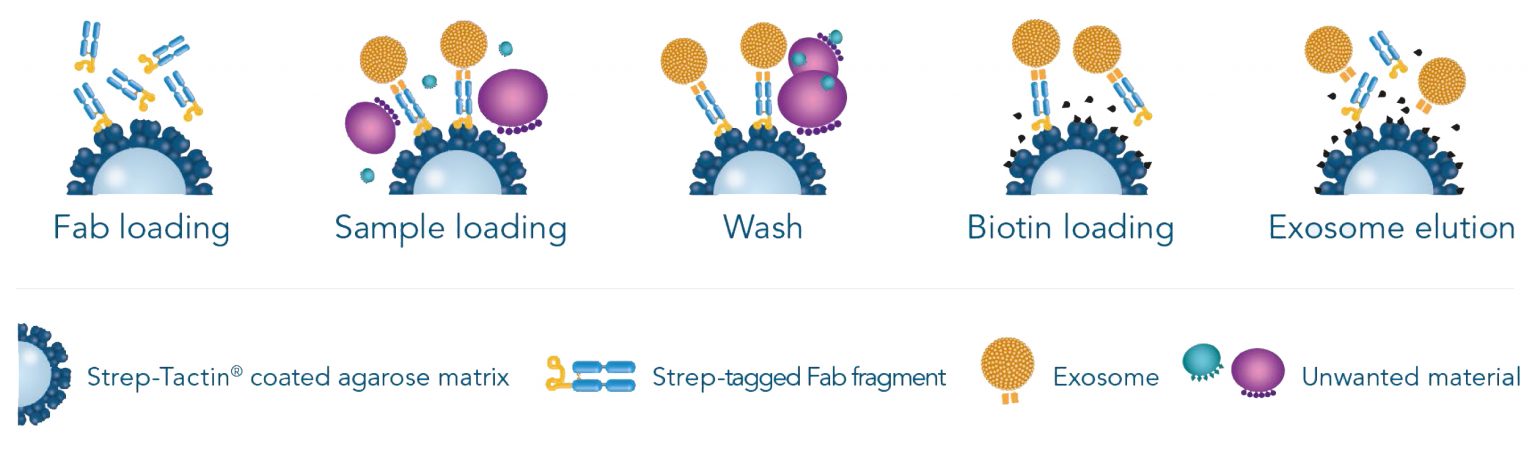
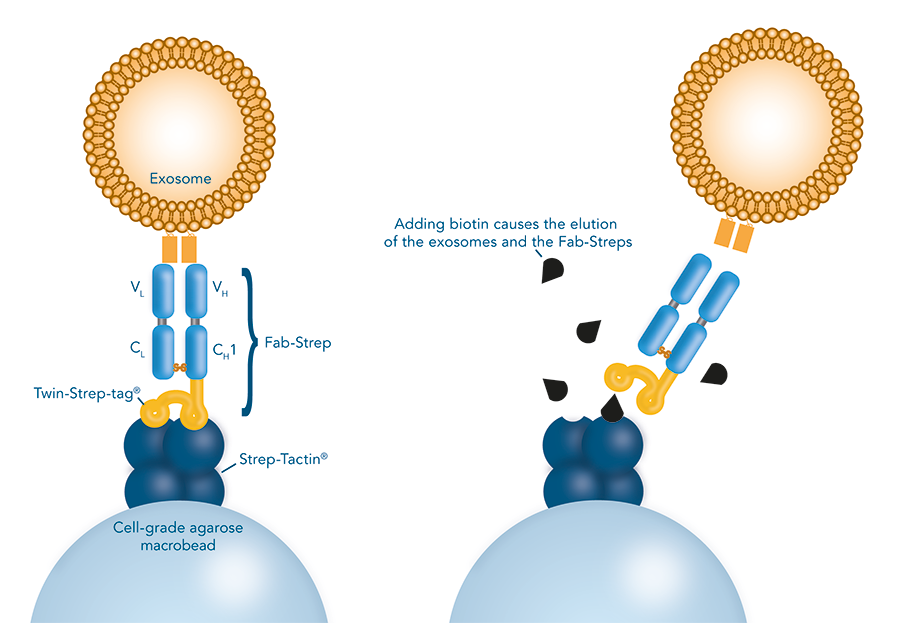
Columns for exosomes are filled with a Strep-Tactin® coated matrix made of cell-grade agarose. Strep-tagged low affinity Fab fragments (Fab-Streps) specifically bind to the matrix. Subsequently, cell culture supernatants, serum, plasma or other preparations pass through the column. Exosomes adhere to the matrix based on the exclusive binding of the Fab-Strep to the target. Unwanted material is efficiently washed away. In a final step, the addition of biotin causes the elution of the exosomes and the Fab-Streps due to the higher affinity of biotin to Strep-Tactin®. After elution, the Fab-Streps self-dissociate from the vesicle surface. The label-free authentic exosomes are now ready for further downstream applications.
In case ultra-pure exosome preparations without Biotin and Fabs are required, size exclusion chromatography or hydrostatic filtration dialysis can be performed.
High Quality Exosme Isolation
Minimal contamination by other EVs
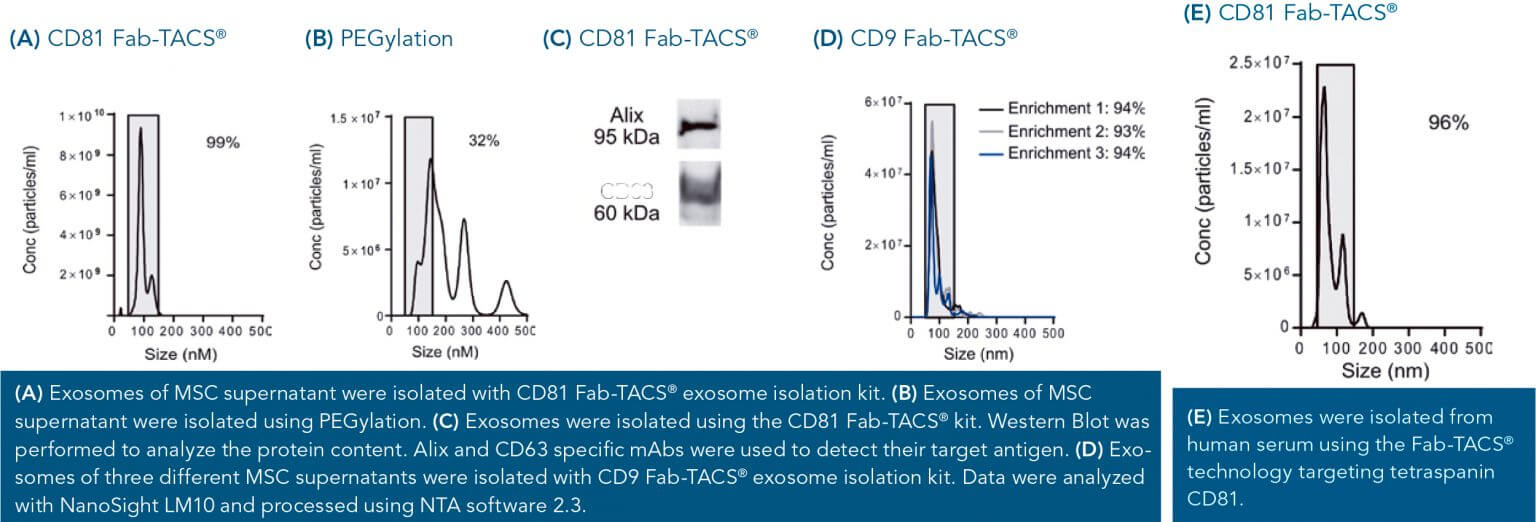
The particle size is a critical factor in evaluating the quality of isolated exosomes. 99% of particles isolated from cell culture supernatants of mesenchymal stem cells (MSCs) fell within the range of 30 – 150 nm (A). This indicates strong exosome enrichment. In comparison, only 32% exosome-sized particles were detected after puri cation with a commercially available PEGylation kit (B), implying contamination by larger extracellular vesicles.
Reproducible results
Depending on MSC donor, cell culture supernatant compositions may vary. Purified exosomes of three independent isolations from different MSC donors were very comparable in their size ranging from 86 nm to 90 nm average diameter. All isolations yielded around 94% of particles between 30 and 150 nm in size (D). Besides high purity, this demonstrates a high reproducibility of our Fab-TACS® exosome isolation technology.
Quick and simple
Due to the surface protein specificity of the Fab-TACS® isolation technology, time-consuming centrifugation steps or extensive sample preparations are not necessary. The protocol is easy and straight-forward, permitting efficient and high quality exosome isolation also for newcomers in the field of exosome research. Additionally, the short processing time minimizes the stress of cargo contained within the exosomes.
High content of exosome proteins
Since other same-sized non-exosome contaminants may contribute to the pool of isolated particles, the vesicles purified by our Fab- TACS® isolation technology from MSC supernatants were tested for the presence of marker proteins CD63 and Alix. Both proteins were clearly present within the purified particles (C), confirming their exosome phenotype.
Source-independent application
Important exosomes sources also include a variety of human bodily fluids such as blood and urine. Similar to isolations from cell culture supernatants, 96% of particles purified from human serum exhibited the typical exosome size of 30 to 150 nm (E).